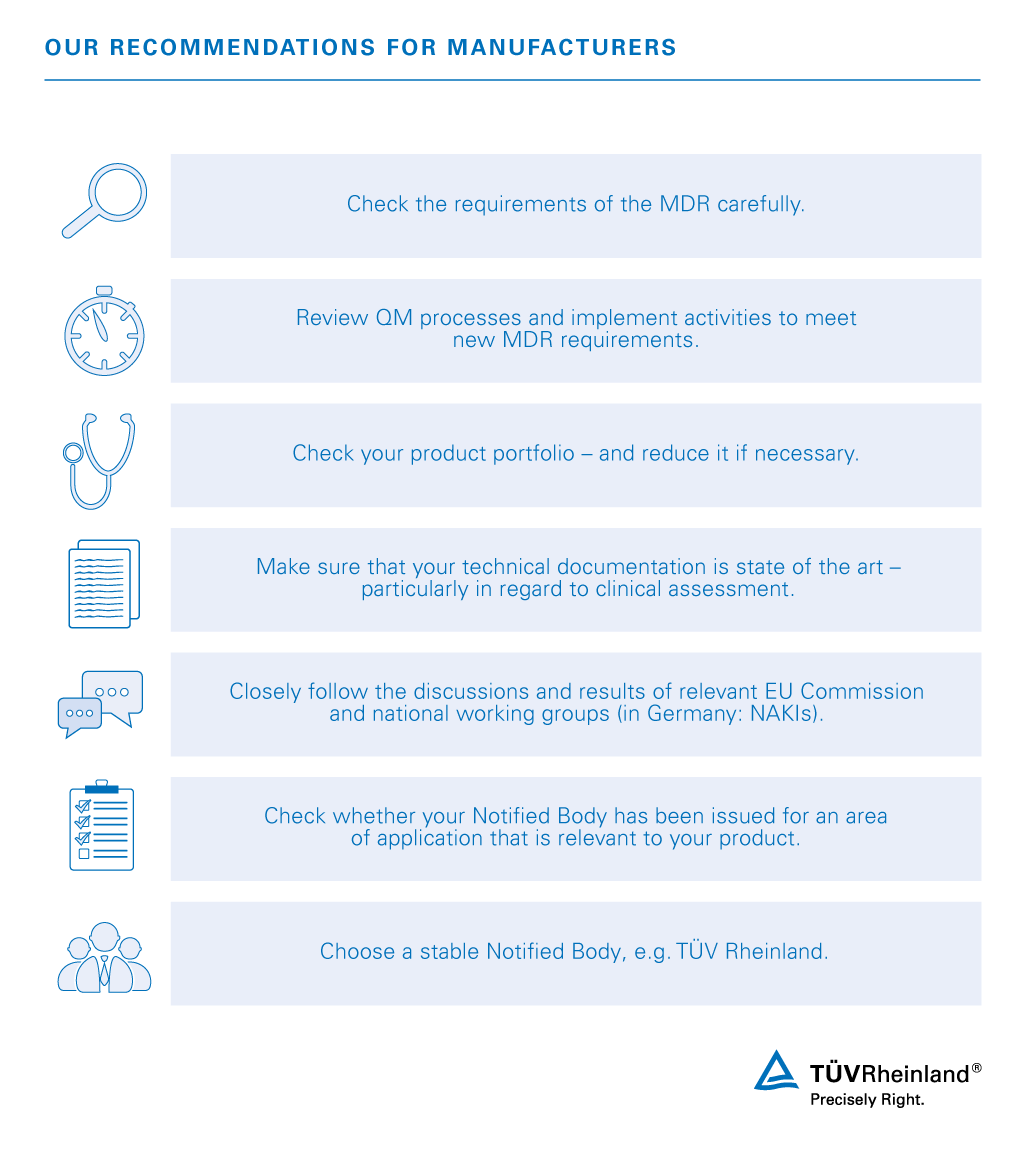
Availability and capacity of notified bodies to carry out conformity assessments for COVID-19 related medical devices and in vit

Mario Gabrielli Cossellu on LinkedIn: #notifiedbody #mdr #nando #ivdr #conformityassessment #eu #regulations… | 11 comments